How Do You Build ISO/IEC 17025 Ready Labs with End-to-End Digital Traceability?
Author

Neerav Singh
Technical Product Specialist

Author

Neerav Singh
Technical Product Specialist
Reading Time
4 min read
- Why ISO 17025 Alignment Matters More Than Ever?
- Proof Is the New Output.
- When Manual Compliance Starts to Crack?
- What ISO/IEC 17025 Is Really Evaluating?
- Passing Audits vs. Running a High-Performance Lab
- When the Test Lifecycle Is Fully Connected with Digital Traceability
- How Different Labs Experience the Impact?
- The Takeaway: ISO 17025 as a Competitive Advantage
How Do You Build ISO/IEC 17025 Ready Labs with End-to-End Digital Traceability?
If you run or work with testing and calibration laboratories whether as an OEM lab, an independent testing facility, or an internal validation team, you’ve probably heard this question more times than you can count:
“Is your lab ISO/IEC 17025 aligned?”
At first glance, it sounds simple. But behind that question lies a much larger shift in how modern labs are expected to operate. Today’s laboratories are distributed, digital, fast-moving and under constant scrutiny from customers, regulators and leadership.
That’s exactly why ISO/IEC 17025 exists.
Often referred to casually as ISO 17025, this international standard sets out the benchmark for technical competence in ISO-aligned testing laboratories and calibration facilities. And when understood correctly, it’s far more than a documentation exercise; it’s a framework for trust.
Why ISO 17025 Alignment Matters More Than Ever?
ISO/IEC 17025 defines whether a laboratory can consistently produce technically valid and defensible results. In practical terms, it answers three critical questions:
- Can this lab generate accurate, repeatable test results?
- Are those results traceable, auditable and compliant with defined procedures?
- Is there end-to-end proof that the right processes, people and equipment were used?
The scope of ISO 17025 requirements is intentionally broad. It covers everything from test planning and execution to equipment calibration standards, data integrity, approvals, reporting and record retention.
This is why ISO support for testing laboratories has become non-negotiable. Customers and regulators don’t just want results; they want proof.
Proof Is the New Output.
Modern audits no longer tolerate reconstruction after the fact. Auditors expect clear answers, instantly:
- Who performed the test?
- On which equipment?
- Using which procedure and revision?
- When was the equipment last calibrated?
- Who reviewed and approved the results?
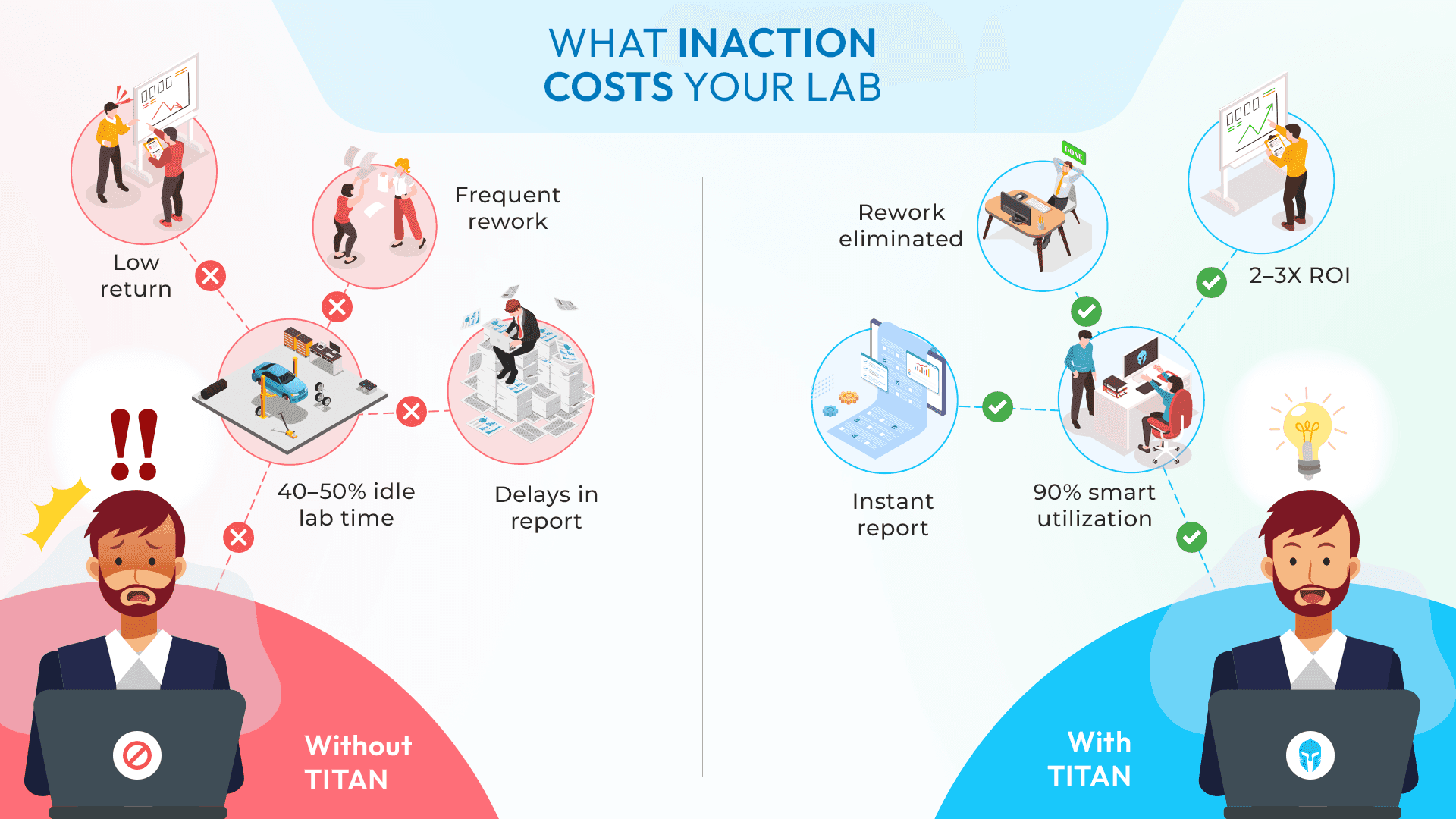
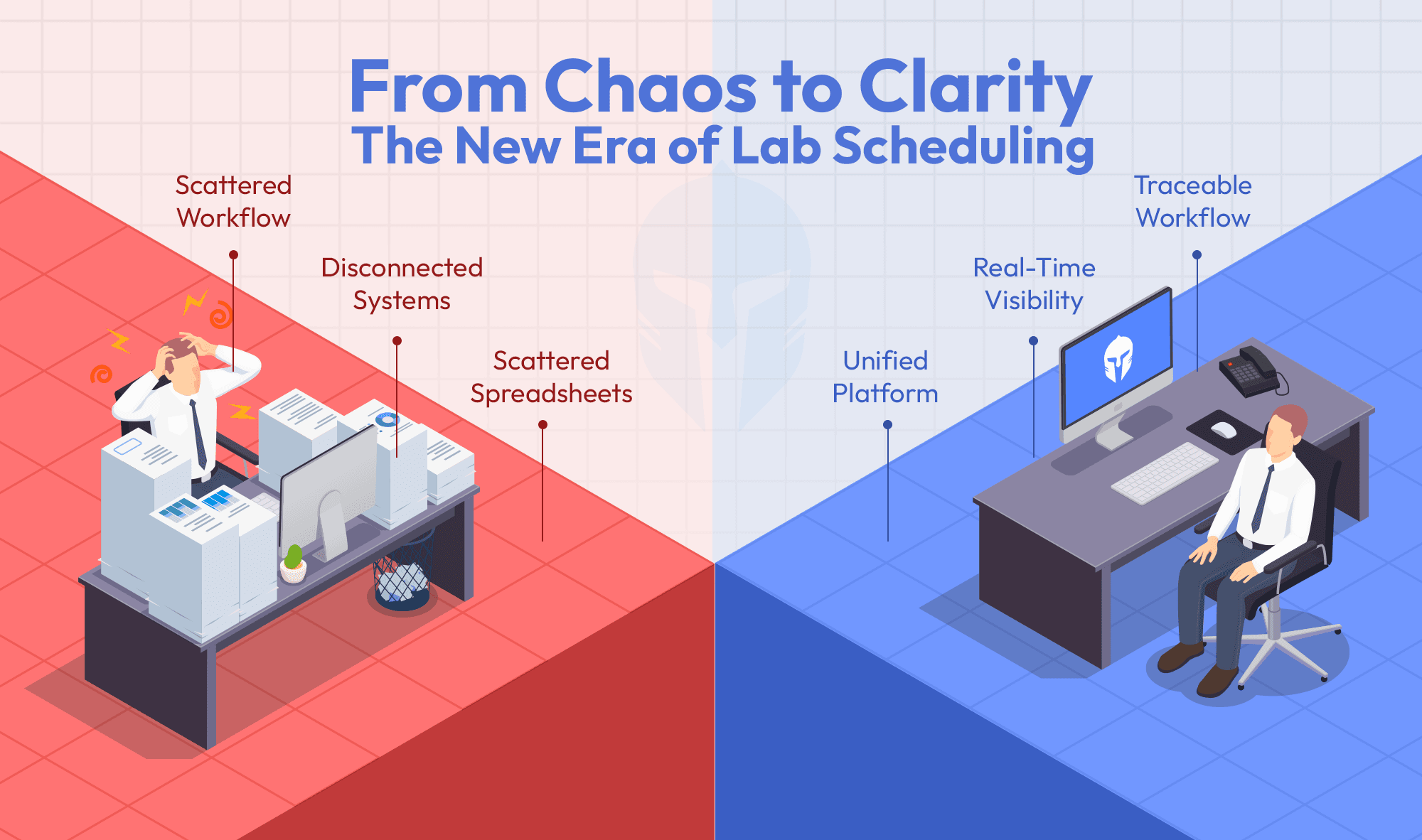
In labs where data lives in emails, spreadsheets or personal folders, traceability breaks down quickly. ISO/IEC 17025 doesn’t allow “best effort.” It requires systematic control regardless of lab size or geography.
When Manual Compliance Starts to Crack?
Many labs try to meet ISO 17025 requirements through manual processes. Over time, this approach introduces risk:
- Missed or incomplete checklists
- Inconsistent reporting formats
- Delayed or undocumented approvals
- Fragmented audit trails
This leads to a common misunderstanding: that ISO 17025 is about paperwork.
In reality, ISO/IEC 17025 tests operational visibility.
What ISO/IEC 17025 Is Really Evaluating?
Strip away the formal language and the standard comes down to a few practical expectations:
One Test, One Complete Story
From request to final report, every step must connect, planning, execution, results, approvals and reporting. Nothing should exist in isolation.
Repeatable Processes, Not Tribal Knowledge
Compliance can’t depend on who’s running the test. ISO 17025 expects enforced workflows that guide every user, every time.
If It’s Not Recorded, It Didn’t Happen
Approvals, changes, timestamps and justifications must be backed by data, not memory. Samples and equipment are uniquely identified through barcode labels to support traceability and minimize the risk of misidentification.
Calibration Standards That Protect Result Validity
Equipment calibration and preventive maintenance must be directly linked to testing schedules. In testing and calibration laboratories, equipment readiness is essential as it directly affects the reliability of results.
Passing Audits vs. Running a High-Performance Lab
Many labs aim for one thing: passing audits.
High-performing labs aim higher. They use ISO/IEC 17025 as a foundation for daily operational excellence. When done right, compliance leads to:
- Stronger consistency
- Faster reporting
- Smarter resource utilization
- Better decision-making
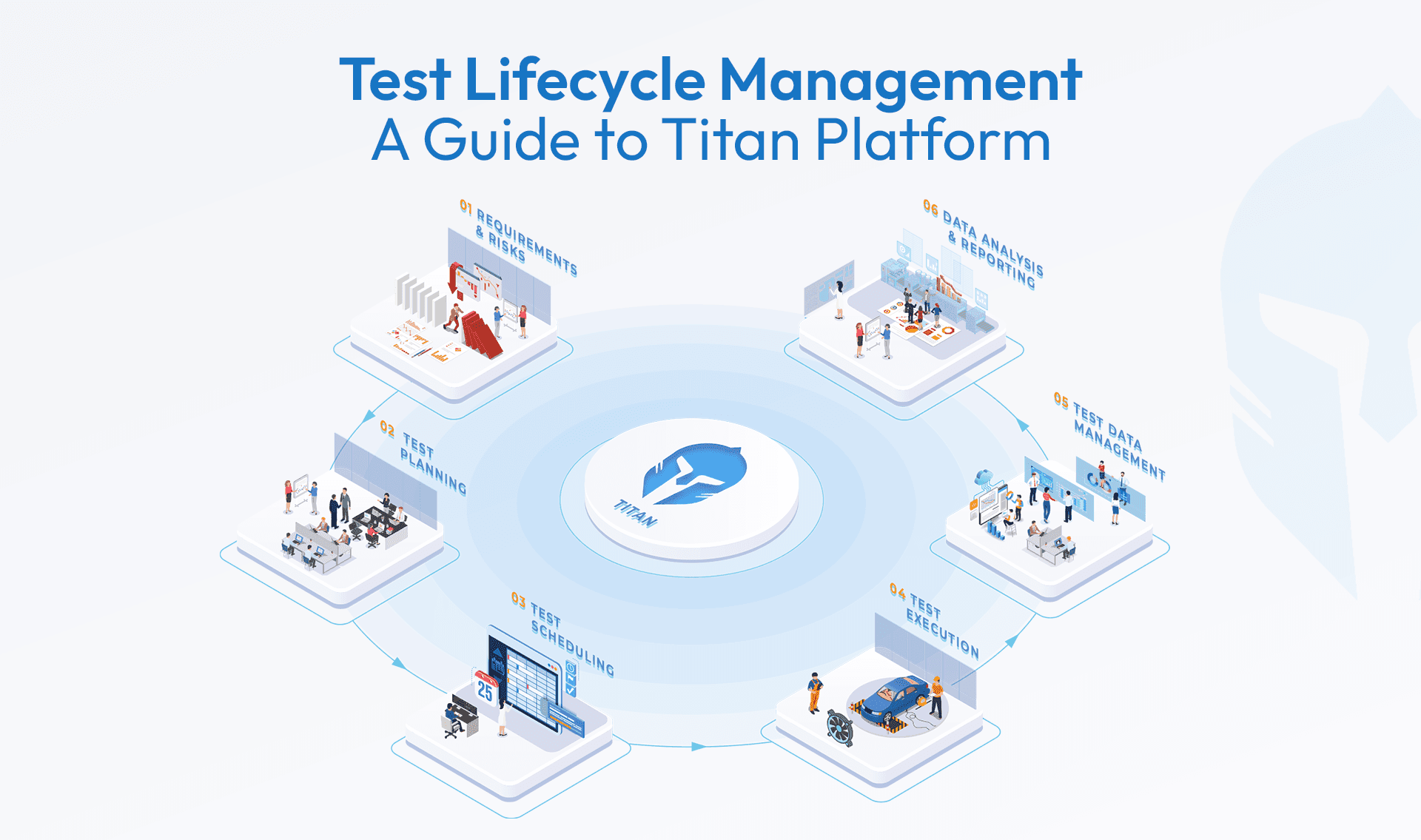
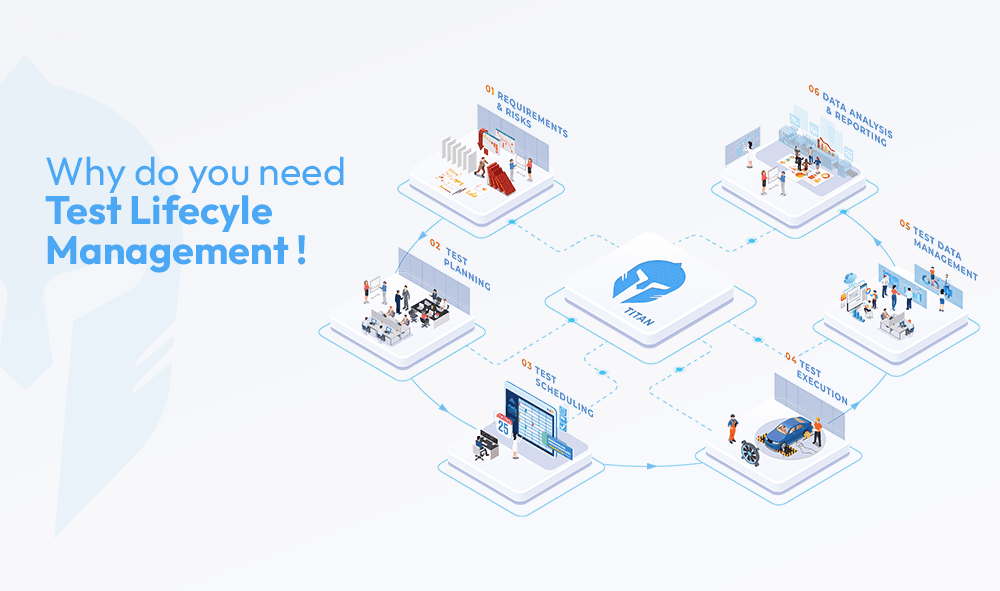
This is where digitally connecting the entire testlifecycle changes everything.
When the Test Lifecycle Is Fully Connected with Digital Traceability
A unified, digital approach creates a single thread across people, processes, equipment and data. That thread delivers exactly what ISO 17025 alignment demands:
- Built-in traceability through audit trails, electronic signatures and real-time linking of every action to timestamps, users and standards
- Audit-ready records by default, with tamper-proof logs that eliminate manual reconstruction and ensure data integrity
- Real-time visibility instead of last-minute scrambling, enabling instant answers to auditor questions and proactive risk management
Compliance stops being an event. It becomes the way work naturally happens. Digital traceability ensures unbroken chains of evidence for measurements, calibrations and decisions, aligning perfectly with the standard emphasis on traceability, control of records and information management.
How Different Labs Experience the Impact?
OEM Testing Laboratories
Standardized execution, integrated calibration schedules and clear ownership lead to consistent outcomes across teams and locations.
Leadership Teams
Centralized visibility replaces guesswork, enabling data-driven decisions on capacity, bottlenecks and priorities.
Independent Testing and Calibration Laboratories
Automation reduces manual effort while improving consistency and audit readiness, boosting throughput without increasing engineering load.
The Takeaway: ISO 17025 as a Competitive Advantage
ISO/IEC 17025 stands for reliable and disciplined testing.
A signal that your lab’s results can be trusted.
That your calibration standards are under control. That your processes are built to scale.
Compliance alone is just the starting line. The labs that truly succeed treat ISO 17025 alignment as a living system digitally traceable, operationally disciplined and ready for whatever audit, customer or challenge comes next.
That’s when compliance stops being stressful and starts being powerful.
Understand Your ISO/IEC 17025 Readiness
Assess how your testing and calibration processes align with ISO/IEC 17025.